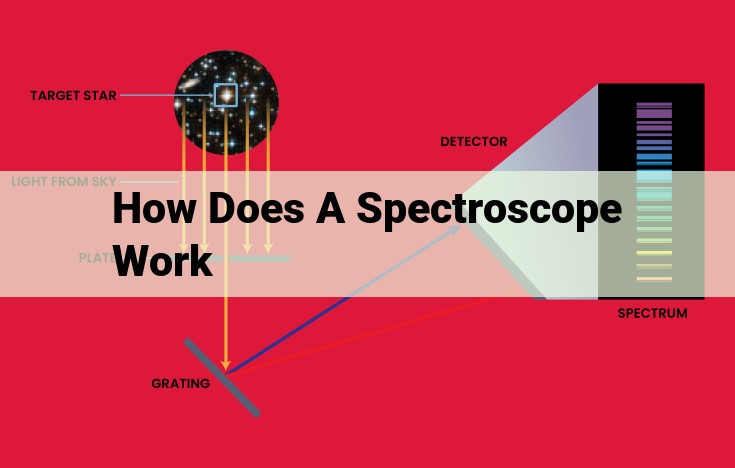
Light is spread into a spectrum when passed through a prism or grating in a spectroscope. By analyzing the resulting pattern of wavelengths, spectroscopes identify the composition of a substance. The instrument separates light based on its wavelengths, revealing the distinct absorption or emission lines characteristic of different elements or molecules. This enables scientists to determine the chemical composition, structure, and properties of materials.
Spectroscopy: Unveiling the Secrets of Matter
In the realm of science, spectroscopy emerges as a powerful analytical tool that allows us to peek into the inner workings of matter. It’s like a microscope that, instead of magnifying objects in space, unravels the secrets of their composition and structure.
What is Spectroscopy?
Spectroscopy is the study of the interaction between light and matter. When light strikes matter, it can be absorbed, emitted, or scattered. The pattern of this interaction depends on the wavelength of the light and the nature of the matter. By analyzing these patterns, spectroscopists can gain valuable insights into the composition, structure, and properties of materials.
Types of Spectroscopic Techniques
There are numerous spectroscopic techniques, each designed to probe specific aspects of matter’s behavior. Some common techniques include:
- Atomic emission spectroscopy: Analyzes the patterns of light emitted by atoms when they’re excited
- Molecular spectroscopy: Examines the absorption or emission of light by molecules
- Ultraviolet-visible spectroscopy: Measures the absorption or reflection of ultraviolet and visible light by substances
- Infrared spectroscopy: Explores the absorption or emission of infrared radiation by materials
- Nuclear magnetic resonance (NMR) spectroscopy: Utilizes magnetic fields and radio waves to study the structure and dynamics of molecules
Components and Types of Spectroscopes
The heart of every spectroscopic technique lies in the spectroscope, a device that separates light into its component wavelengths. Understanding its intricate workings is essential for unraveling the secrets of the universe and unlocking the mysteries hidden within materials.
At its core, a spectroscope comprises three fundamental components: the collimator, the dispersing element (prism or grating), and the eyepiece/camera.
The Collimator: Guiding the Light’s Path
The collimator, acting as the gatekeeper of light, transforms a beam of divergent rays into a parallel bundle. This crucial step ensures that light enters the dispersing element uniformly, setting the stage for accurate spectral analysis.
The Dispersing Element: Unraveling the Rainbow
The dispersing element, either a prism or a grating, plays a pivotal role in separating light into its constituent wavelengths. Prisms, with their inherent refractive properties, bend light to different extents based on its color, resulting in a spectrum. Gratings, on the other hand, utilize the principles of diffraction to achieve the same separation, creating a series of interference patterns that reveal the spectral components.
The Eyepiece/Camera: Capturing the Spectrum
The final stage of the spectroscope’s journey is the eyepiece or camera. The eyepiece allows the observer to visually inspect the dispersed light, while the camera serves as a digital recorder, capturing the spectrum for further analysis and interpretation.
Prism vs. Grating Spectroscopes: A Tale of Two Dispersers
While both prism and grating spectroscopes share the common goal of spectral separation, they employ distinct mechanisms to achieve this. Prisms rely on the principle of refraction, bending light based on its wavelength, while gratings utilize the phenomenon of diffraction, creating a series of interference patterns.
Prism spectroscopes, renowned for their high resolution and ability to resolve fine details, are often employed in applications requiring precise spectral measurements. However, gratings offer a wider wavelength range, compactness, and cost-effectiveness, making them a popular choice for various spectroscopic techniques.
Atomic Emission, Molecular Spectroscopy, and Absorption Spectroscopy
- Explanation of each technique and its applications
Atomic Emission Spectroscopy
Imagine this: you’re holding a match under a clear night sky. The flame dances and glows, emitting a shimmering hue that tells a tale of the elements within. This is atomic emission spectroscopy at work.
When atoms absorb energy, their electrons jump to excited energy levels. Upon returning to their ground state, they release the absorbed energy as light of specific wavelengths. By analyzing these wavelengths, scientists can identify the elements present in the sample, as each element has its unique spectral fingerprint.
Molecular Spectroscopy
Shifting focus from atoms to molecules, molecular spectroscopy sheds light on the structure and composition of compounds. Molecules vibrate and rotate, absorbing energy at specific frequencies. These frequencies correspond to the molecular bonds and functional groups, allowing scientists to determine the molecule’s identity and structural characteristics.
Absorption Spectroscopy
Now, let’s consider the opposite of emission: absorption spectroscopy. Instead of atoms emitting light, they absorb it. When light of a specific wavelength is passed through a sample, certain wavelengths are absorbed by the atoms or molecules present. By measuring the absorbed wavelengths, scientists can identify and quantify the components in the sample.
Applications of these Techniques
These spectroscopic techniques play crucial roles in various fields:
- Chemical Analysis: Identifying and quantifying elements and compounds in samples.
- Material Characterization: Determining the composition, structure, and properties of materials.
- Astronomy: Analyzing starlight and interstellar matter to study celestial objects.
- Medical Diagnostics: Identifying diseases and analyzing biomolecules in blood and tissues.
- Environmental Monitoring: Detecting pollutants and assessing air and water quality.
Spectroscopy: Unveiling the Secrets of Matter through Light
Spectroscopy is an indispensable tool in the realm of chemical analysis, providing unparalleled insights into the composition and structure of substances. By harnessing the interaction of light with matter, spectroscopists can decipher the secrets hidden within atoms and molecules.
Elemental Identification: A Fingerprint of Matter
One of the most fundamental applications of spectroscopy is elemental identification. Each element possesses a unique set of spectral lines, akin to a fingerprint. These lines arise from the absorption or emission of specific wavelengths of light, providing a definitive identification of the elements present in a sample.
Molecular Spectroscopy: Unveiling Structural Secrets
Beyond elemental analysis, spectroscopy also empowers chemists to unravel the molecular structure of compounds. By studying the characteristic vibrations and rotations of molecules, spectroscopists can deduce the presence of specific functional groups, bond lengths, and molecular geometry. This information is vital for understanding the chemical reactivity, properties, and behavior of substances.
Quantitative Analysis: Precision Measurement
Spectroscopy is not limited to qualitative analysis; it also enables the precise quantification of chemical species. By measuring the intensity of spectral lines or bands, spectroscopists can determine the concentration of specific elements or compounds within a sample. This quantitative information is crucial for controlling industrial processes, monitoring environmental contaminants, and conducting medical diagnostics.
Spectroscopy: A Powerful Tool for Material Characterization
Spectroscopy, a fascinating realm of science, empowers us to unravel the hidden secrets of materials. By analyzing the interactions between light and matter, we embark on a journey of discovery, unlocking profound insights into the composition, structure, and properties of materials.
Surface Analysis: Probing the Outermost Layer
Spectroscopic techniques provide a window into the surface of materials, revealing their outermost layer with remarkable precision. Scanning Electron Microscopy with Energy-Dispersive X-ray Spectroscopy (SEM-EDS) is a potent tool in this regard. It bombards the surface with high-energy electrons, releasing X-rays that unveil the elemental makeup of the material.
Composition Determination: Identifying Building Blocks
Spectroscopy illuminates the composition of materials, identifying the atoms that form their molecular structure. Atomic Emission Spectroscopy (AES) and X-ray Fluorescence (XRF) are valuable techniques for this purpose. AES vaporizes atoms and analyzes their emitted light, while XRF harnesses X-rays to excite and identify the elements present.
Structural Characterization: Unveiling the Fabric of Materials
Delving deeper, spectroscopy unravels the intricacies of material structure. X-ray Diffraction (XRD) and Infrared Spectroscopy (IR) provide invaluable insights in this arena. XRD bombards materials with X-rays to determine their crystal structure, while IR analyzes the absorption of infrared radiation, revealing molecular bonds and functional groups.
By harnessing the power of spectroscopy, we gain an unprecedented understanding of materials, from their surface composition to their internal structure. This knowledge empowers us to develop innovative materials with tailored properties, drive scientific advancements, and solve complex challenges across a myriad of industries.
Spectroscopic Techniques in Astronomy: Unraveling the Secrets of the Cosmos
Astronomy, the study of celestial bodies, has been transformed by the advent of spectroscopy, a powerful technique that allows scientists to analyze the light emitted or absorbed by stars, planets, and interstellar matter. Spectroscopy provides invaluable insights into the composition, structure, and dynamics of cosmic objects.
Analyzing Starlight
By analyzing the light emitted by stars, astronomers can determine their temperature, chemical composition, and physical properties. The different wavelengths of light emitted or absorbed by a star reveal the presence of specific elements, such as hydrogen, helium, and ionized metals.
Exploring Interstellar Matter
Spectroscopy also enables the exploration of interstellar matter, the gaseous and dusty material that permeates the vastness of space. By studying the light absorbed and re-emitted by interstellar clouds, scientists can determine their density, temperature, and chemical composition. This information sheds light on the formation and evolution of stars and galaxies.
Classifying Celestial Objects
Spectroscopy plays a crucial role in classifying celestial objects. The distinct spectral signatures of different types of stars, such as main sequence stars, red giants, and white dwarfs, allow astronomers to identify and categorize them based on their stellar evolution. Similarly, the chemical composition of interstellar clouds can reveal their origin and associated celestial objects.
Revealing Cosmic Dynamics
Spectroscopy provides insights into the dynamics of celestial objects. By studying the Doppler shift in starlight, astronomers can determine the velocity of stars and galaxies relative to Earth. This information helps them understand the expansion of the universe and the motion of celestial bodies within it.
Expanding our Cosmic Understanding
Spectroscopic techniques have revolutionized our understanding of the cosmos. They have enabled astronomers to explore the nature and evolution of stars, unravel the secrets of interstellar matter, classify celestial objects, and probe the dynamics of the universe. As we delve deeper into the vastness of space, spectroscopy remains an indispensable tool for unraveling the profound mysteries that lie beyond our planet.
Medical Diagnostics Applications of Spectroscopy
Spectroscopy, the study of the interaction between light and matter, has revolutionized the field of medical diagnostics. Its diverse techniques empower clinicians to analyze biological samples non-invasively, leading to accurate diagnoses and improved patient outcomes.
Blood Analysis
Spectroscopic techniques, such as ultraviolet-visible (UV-Vis) and infrared (IR) spectroscopy, provide valuable insights into blood composition. They can detect anomalies in hemoglobin levels, indicative of anemia or polycythemia. Spectroscopic analysis also aids in diagnosing metabolic disorders, such as diabetes and kidney disease, by measuring glucose and urea levels.
Tissue Identification
Spectroscopy has become indispensable in biopsy procedures. It facilitates the identification of specific cell types and tissues, aiding in the diagnosis of various diseases, including cancer. For instance, Raman spectroscopy allows real-time identification of tissues during surgery, minimizing the need for invasive biopsies.
Disease Detection
Spectroscopy offers a sensitive and specific approach to disease detection. Techniques like near-infrared (NIR) and fluorescence spectroscopy can detect tumor markers in blood or urine, enabling early diagnosis and monitoring of cancer. Additionally, microwave spectroscopy has shown promise in diagnosing diseases like tuberculosis and HIV.
In summary, spectroscopic techniques have transformed medical diagnostics, providing non-invasive and precise methods for blood analysis, tissue identification, and disease detection. These advancements have significantly improved patient care and opened doors to novel diagnostic approaches in modern medicine.
Environmental Monitoring with Spectroscopy: Unlocking Nature’s Secrets
Spectroscopy, a powerful tool in analytical chemistry, has emerged as a critical technique for safeguarding our planet. By analyzing the interaction of light with matter, spectroscopists can detect and monitor pollutants, ensuring the health of our air and water resources.
Unveiling Hidden Pollutants
Spectroscopy’s ability to identify and measure the concentration of substances makes it an invaluable asset in environmental monitoring. It can detect a wide array of pollutants, including heavy metals, volatile organic compounds (VOCs), and toxic gases, even in trace amounts. This information enables scientists and policymakers to pinpoint sources of pollution, track their movement, and implement effective mitigation strategies.
Guardians of Air Quality
Spectroscopy plays a pivotal role in maintaining clean air. Continuous air monitoring stations employ sophisticated spectrometers to measure pollutant levels in real-time. By analyzing the absorption or emission spectra of pollutants, these devices provide early warnings of potential air quality hazards. This information empowers citizens and governments to take precautionary measures, reducing exposure to harmful airborne pollutants.
Sentinels of Water Purity
Water quality monitoring is another crucial area where spectroscopy shines. It allows scientists to detect contaminants in water, such as heavy metals, pesticides, and industrial chemicals. By identifying the specific pollutants present, water treatment plants can optimize their purification processes, ensuring the safety of our drinking water. Spectroscopy also plays a key role in monitoring water bodies for pollution, protecting aquatic ecosystems and safeguarding the health of both humans and wildlife.
Spectroscopy: A Vital Tool for Environmental Protection
In conclusion, spectroscopy has become an indispensable tool in environmental monitoring, providing us with the ability to detect and measure pollutants with unprecedented accuracy. By uncovering the secrets hidden within light’s interaction with matter, spectroscopy empowers scientists, policymakers, and citizens alike to safeguard our planet and ensure the well-being of future generations.
Key Concepts in Spectroscopy
Wavelength, Frequency, and Energy
Light is a form of electromagnetic radiation, which consists of waves of oscillating electric and magnetic fields. The distance between two consecutive peaks or troughs of the wave is known as the wavelength, denoted by the Greek letter lambda (λ). The number of complete waves that pass a fixed point in one second is called the frequency, denoted by the letter nu (ν). Both wavelength and frequency are inversely proportional, meaning as one increases, the other decreases.
The energy of a photon, the basic unit of light, is directly proportional to its frequency. Higher energy photons have shorter wavelengths and higher frequencies. The electromagnetic spectrum, which includes all forms of electromagnetic radiation, is arranged in order of increasing energy, with radio waves at the lowest energy and gamma rays at the highest.
Interaction of Light with Matter
When light interacts with matter, it can undergo various processes, including:
- Reflection: Bouncing off a surface, like a mirror.
- Transmission: Passing through a transparent material, like glass.
- Absorption: Being absorbed by the material, which can result in changes in its electronic structure.
- Scattering: Deflecting in all directions, like light from particles in smoke.
Spectroscopy focuses on the absorption and emission of light by atoms and molecules. When light of the correct energy is absorbed, it can excite electrons within the atom or molecule, causing them to move to higher energy levels. When the electrons return to their original energy levels, they release the absorbed energy as light, often of a different wavelength.
Quantum Mechanics and Its Role in Spectroscopy
Quantum mechanics is the branch of physics that describes the behavior of matter at the atomic and subatomic levels. It introduces the concept of quantization, where energy is not continuous but exists in discrete, well-defined levels. The absorption and emission of light in spectroscopy can only occur at specific wavelengths corresponding to the energy differences between allowed quantum energy levels.
Understanding the quantum mechanical nature of light-matter interactions is essential for interpreting spectroscopic data and gaining insights into the structure and properties of atoms and molecules. Spectroscopy provides a powerful tool for exploring the subatomic world, revealing the fundamental nature of matter and its interactions with light.